Welcome to the site of the Laboratory of Chemical, Electrochemical & Particle Processes (LCEPP)
The Laboratory of Chemical, Electrochemical and Elementary Particle Processes, founded by Professor Emeritus Constantinos G. Vayenas, and directed by Professor Alexandros Katsaounis, belongs to the Department of Chemical Engineering at the University of Patras. The group of Patras is credited with the discovery of the effect of Electrochemical Promotion of Catalysis (EPOC) and has pioneered in the use of solid electrolytes, as an active reservoir of ionic species available to control and enhance the catalytic properties of metal and metal oxide electrodes.
The group has an extended experience in catalytic and electrocatalytic processes, which is shown by almost 10 scientific publications per year in high citation impact journals (Journal of Catalysis, Journal of Electrochemical Society, Journal of Applied Catalysis). The group has more than one hundred publications both on the phenomenology and on the fundamentals of EPOC. Research is also being carried out on the use of fuel cells with alternative fuels for simultaneously generation of electrical power and useful chemicals (‘’chemical cogeneration’’). The group of LCEPP has also pioneered recently the use of triode fuel cells where a third auxiliary electrode is used to enhance the anodic or cathodic electrocatalysis. Among latest achievements is the development of the monolithic electropromoted reactor (MEPR) which significantly facilitates the practical utilization of electrochemical promotion of catalysis.
A. Current research in Catalytic and Electrochemical Processes:
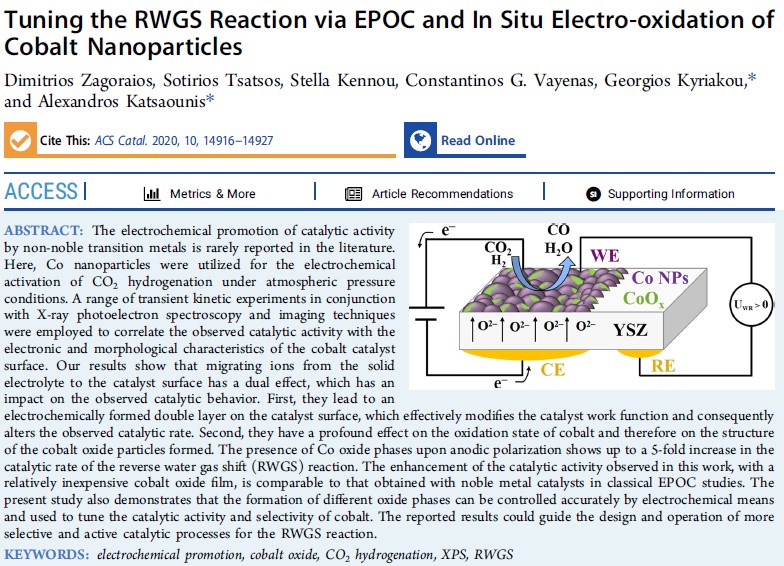
- Electrochemical Promotion of CO2 hydrogenation to high value chemicals and fuels.
- Effect of carbon support (graphene, carbon nanotubes, biochar, vulcan etc.) on the performance of anodic and cathodic electrodes for PEMFCs
- Electrochemical Promotion of catalytic reactions in aqueous media.
- Preparation, characterization and development of electrochemical sensors for phenolic compounds in wastewater
B. Current research in Particle Physics:
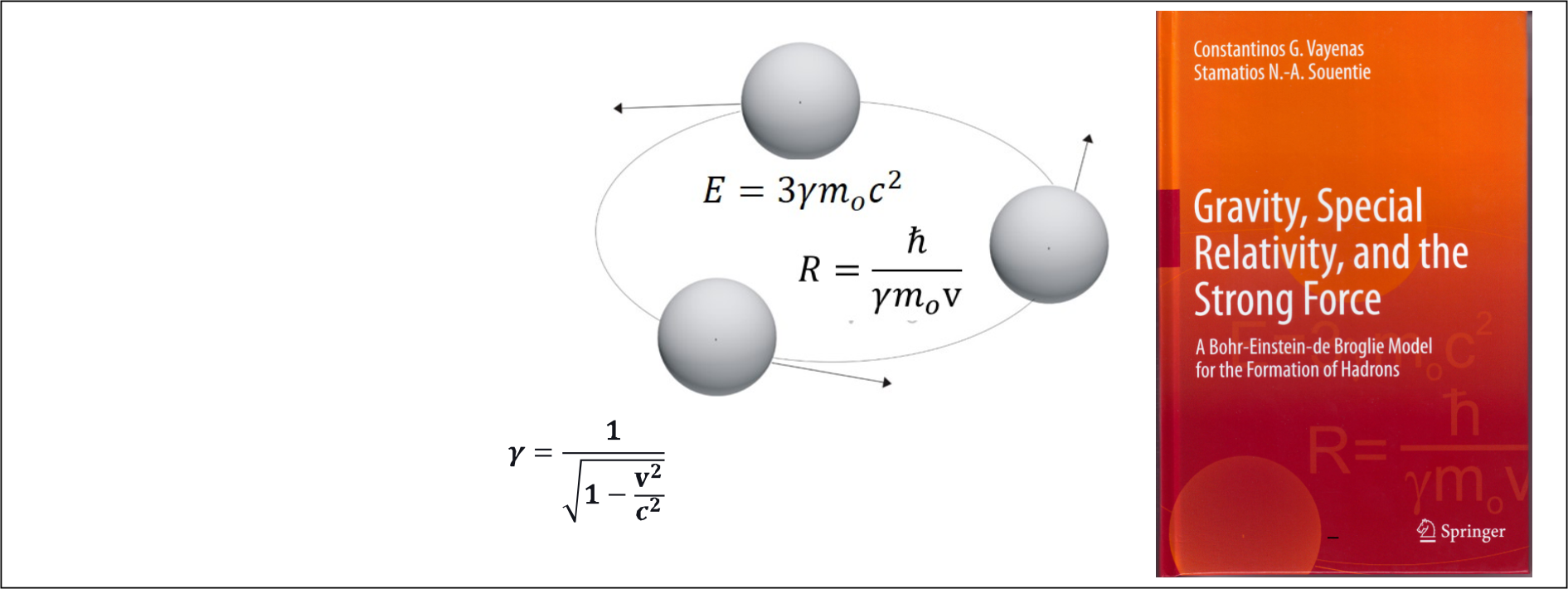
- Application of Special and General Relativity in Particle Physics
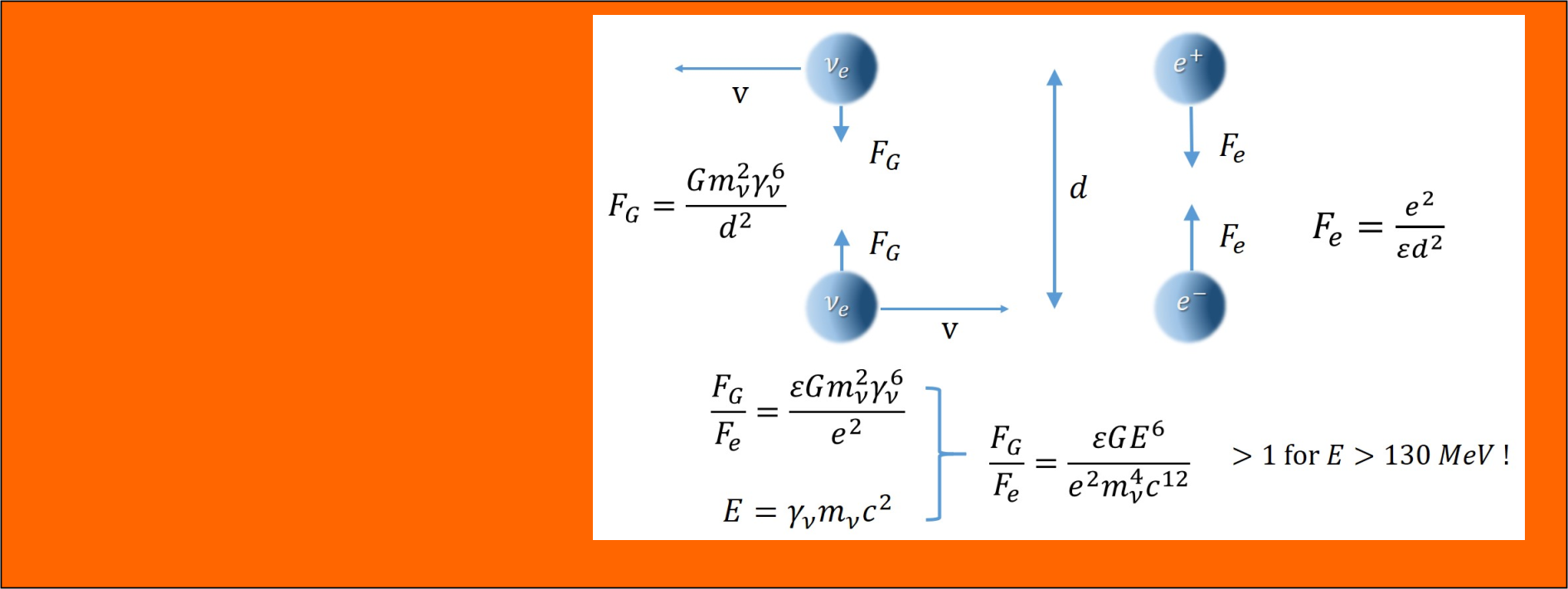
- Gravitational interaction between neutrinos
- Gravitational confinement of neutrinos
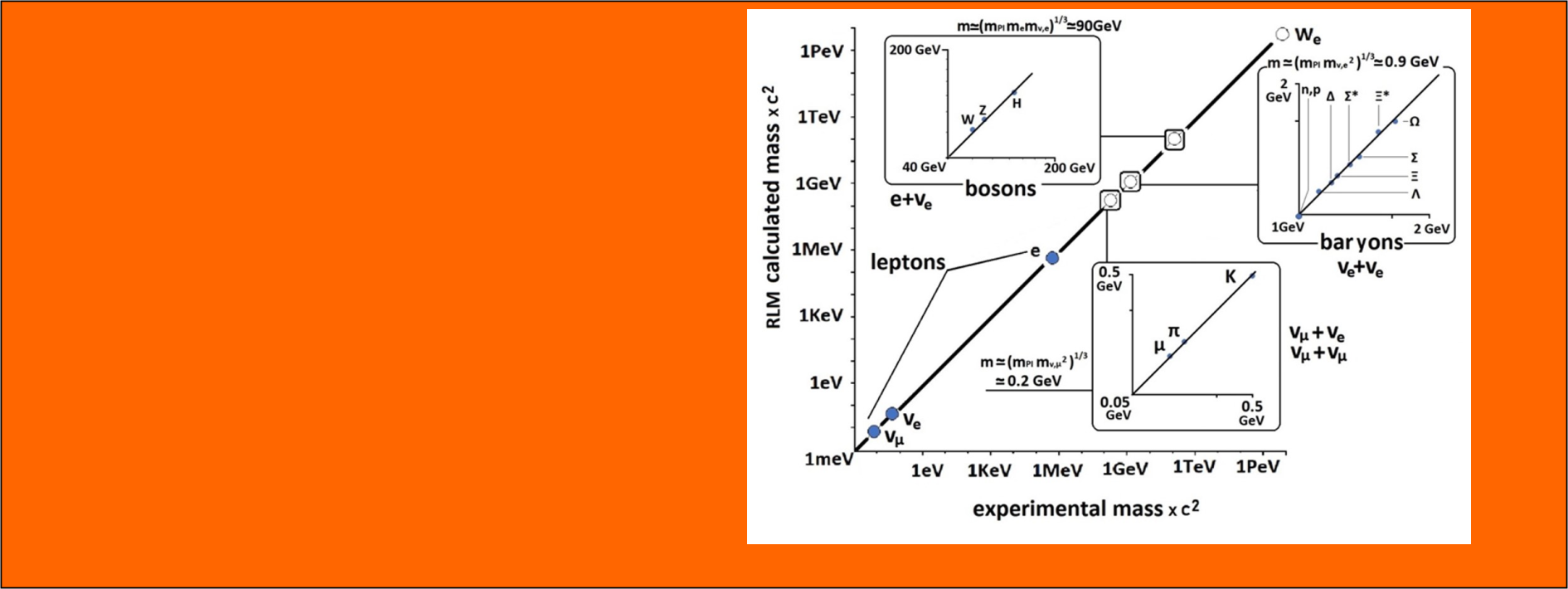
- Mechanism of mass generation via gravitational confinement
- Analytical computation of the masses of hadrons and bosons
- Study of the role of gravity in elementary particle systems
Latest news
01
18
18
Open Positions
- no available diploma thesis positions
- no available phD thesis positions